Sorghum is most susceptible to crop loss from insect pests during flowering and grain fill, and this article deals mostly with these species (sorghum midge, helicoverpa and Rutherglen bug).
However, some years there may be pest infestations in vegetative sorghum.
Vegetative sorghum
During the vegetative stages you may see a few armyworm or helicoverpa larvae causing shot-holes in the leaves, but this damage is generally just cosmetic. Corn aphids tend to disappear by the time heads have emerged; they are very vulnerable to predation and parasitism, particularly once they are no longer protected within the whorl.
On rare occasions, large helicoverpa larvae can move from leaf feeding (or feeding on weeds) up onto heads at late flowering-early grain fill. Because these larvae are unexpected, and larger than can be effectively controlled by NPV (Vivus®), they can cause significant damage. Thorough and regular crop monitoring will pick these up. Large helicoverpa larvae tend to graze on filling grains, and the damage can be a clue to their presence.
Reproductive sorghum
Monitor for Helicoverpa, sorghum midge and Rutherglen bug (RGB) from head emergence.
Key points:
- Egg parasitism in helicoverpa can be very high, particularly in fields that have not been sprayed
- Johnson grass abundance in late spring can be an indicator of likely midge pressure in early crops.
- Later planted sorghum is at greater risk of midge attack as it is exposed to larger populations that have built up in earlier crops
- Sorghum is most susceptible to crop loss from RGB during early grain fill. Seed crops are particularly vulnerable as feeding reduces both yield and seed viability.
- It is critical that RGB adult numbers are recorded during flowering and grain fill. In some seasons, repeated immigration of RGB adults can make control decisions difficult.
More detailed information on pest sampling and management options (on this page):
See also the Beatsheet video on Sampling insects in sorghum.
Helicoverpa
Monitoring egg lays
The majority of helicoverpa egg laying occurs on heads as they emerge, and before they start flowering. Eggs can be detected by shaking/beating/spinning sorghum heads into a bucket to look for helicoverpa eggs. Whilst egg counts are not used in the economic threshold decision, they are an indication of the level of moth activity, and potentially subsequent larval pressure. Be aware that many of the eggs laid will not produce damaging larvae.
Eggs are highly susceptible to predation by predatory bugs (damsel bugs, pirate bugs), ants and beetles. Eggs are also frequently parasitised by the tiny Trichogramma wasp. Parasitised eggs turn black and are easily distinguished from newly laid eggs (white) or eggs with developing larvae (brown ring). Egg parasitism can be very high, particularly in fields that have not been sprayed.
Small larvae can be parasitised by larger wasps like Microplitis, which kill the larva before it gets large enough to do significant damage.
Sampling for larvae
Beat/shake/twirl five sorghum heads into a bucket. Count the number of larvae, categorising the larvae by size (less than 7 mm is the critical size for a well targeted NPV treatment). Repeat at a number of sites across the field.
Control of helicoverpa
Nucleopolyhedrovirus (NPV) is the most widely used control option for helicoverpa in sorghum. It is highly effective, and has the benefit of preserving beneficials, which then contribute to the suppression of surviving helicoverpa and aphids in the crop. Peak control is 4-9 days after application, and ongoing infection usually continues through the duration of the crop.
To determine whether the helicoverpa infestation is likely to cause significant economic loss, and treatment is warranted, use the threshold calculator [https://thebeatsheet.com.au/economic-threshold-calculators/].
Getting the best possible result with NPV
Timing
Apply NPV sprays before 50% brown anthers, particularly if the spread of flowering is large. By going early, the early flowering heads will be fully protected and secondary infection will control most caterpillars on the late flowering heads. Target caterpillars less than 7 mm in length. Apply NPV when larvae are actively feeding (25-35°C) to ensure rapid ingestion.
Water quality and volumes
Water used in spray mixes should have a pH of 7. Alkaline water will seriously reduce the performance of NPV, so buffer water with Li700 or equivalent to neutralise pH.
For high-volume, water-based sprays, a minimum of 30 L water/ha is recommended for aerial application, and 100 L water/ha for ground rig application.
Coverage
NPV must be ingested to be effective. Achieving good coverage means paying particular attention to water volumes, nozzles, operating pressure, weather conditions. Spread NPV over as much of the head as possible to ensure caterpillars have a high chance of picking up a lethal dose as they feed.
Other options for control of helicoverpa in sorghum
When well targeted, NPV historically performs very well on grain sorghum, usually achieving greater than 90% control without the use of additives. However, if larval pressure is extreme, even 90% control may not reduce the larval population below the economic threshold (e.g. 10 larvae/head with 90% control still leaves 1 larva/head, which may be above threshold).
So is there another product that will do a better job? The answer is ‘No’. All helicoverpa in sorghum are H. armigera, with high levels of resistance to carbamates and synthetic pyrethroids. The use of these products is likely to produce a result inferior to NPV, and impact adversely on beneficial populations.
Other considerations
Weather, particularly temperature, can slow the rate at which larvae succumb to NPV. If larvae don’t seem to be dying as fast as expected, consider whether the daily average temperature has been cooler than normal.
On average, at 30°C, it takes 4.5 days after infection for a 6-day old caterpillar to die. This compares with 6.2 days at 25°C and 7.5 days at 20°C. Below 20 C, it may take longer than 8 days for larvae to start dying.
Consumption of artificial diet by healthy and NPV-infected 6-day old caterpillars and time to death from NPV at three temperatures (Data from Chris Monsour).
Note that although infected larvae do not die immediately, their consumption (and thus potential damage) declines significantly.
For technical information on the use of NPV visit the AgBitech website.
Sorghum midge
The first generation of sorghum midge occurs in Johnson grass, with midge moving to sorghum in early summer. The abundance of Johnson grass through November and early December can be an indicator of likely midge pressure, particularly in early crops.
Female midge lay eggs into the flower spikelet and the larva feeds on the developing ovary, preventing normal seed from development. The lifecycle of a midge takes just over 2 weeks, so it is possible to have more than one generation in a crop that has flowering staggered over this length of time. Later planted sorghum is at greater risk of midge attack as it is exposed to larger populations that have built up in earlier crops.
Varietal resistance
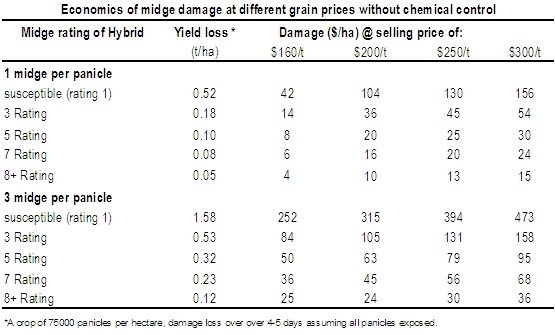
One of the main tools available for managing sorghum midge is midge resistance varieties. The table below shows a rating comparison of potential yield loss under low (1/panicle) and high (3/panicle) midge pressure.
These yield loss estimates assume that (i) spraying results in a 100% kill, (ii) there is no midge damage prior to chemical application, and (iii) average midge pressures remain the same over 4-5 days. In reality, research has shown that one well-timed insecticide for midge (put on from panicle emergence and before midge even enter the crop) will still only prevent 70-80% damage protection in lower-rated sorghum hybrids. In 8 rated hybrids, yield losses can be reduced by over 90%.
Midge rating for current commercial grain sorghum varieties
| Variety | Company | Midge Rating |
|---|---|---|
| HGS-102 | Heritage Seeds | 7 |
| HGS-114 | Heritage Seeds | 6 |
| Cracka | NuSeed | 3 |
| Rippa | NuSeed | 5 |
| Enforcer | NuSeed | 6 |
| Liverty | NuSeed | 4 |
| Tiger | NuSeed | 3 |
| Dominator | NuSeed | 5 |
| MR Buster | Pacific Seeds | 4 |
| MR Bazley | Pacific Seeds | 4 |
| MR Taurus | Pacific Seeds | 6 |
| MR Scorpio | Pacific Seeds | 6 |
| MR Apollo | Pacific Seeds | 7 |
| 84G22 | Pioneer | 4 |
| 85G33 | Pioneer | 6 |
| 85G44 | Pioneer | 4 |
| Agitator | Radicle Seeds | 4 |
| Brazen | Radicle Seeds | 5 |
Sampling for midge
Often the first sign that midge are active is midge caught in spiders’ webs in the field.
Generally, peak midge activity occurs between 9 and 11 am, and this is the best time to look. Monitor for midge over 10 metres of row in at least 4 different locations in your crop.
Changes in weather can bring midge into a field from surrounding areas (Johnson grass, earlier sorghum crops) at any time of day. Midge numbers can vary widely both within a crop and between plants; thorough sampling is critical to estimate midge abundance. Monitor crops daily whilst susceptible.
Sorghum heads are most attractive to midge at mid flower. It is not uncommon to see double or triple the number of midge on panicles at early-mid flower compared with the end of flowering. At lower midge densities, adult flies will move around and lay almost exclusively on the flowering portion of the panicle.
Midge flies are only 1-2 mm long, and it is very easy to underestimate midge numbers if you are not careful. The easiest way to ‘get your eye in’ is to look at the top half of mid flowering panicles and look for MOVEMENT of the small red flies against a still sorghum panicle looking from side on and slightly above side-on one section of the sorghum panicle at a time. Keep your eyes focused over a couple of branches of florets for several seconds at a time to detect female midge walking around the branch or bobbing up and down probing their ovipositor into each floret. On windy days you may have to hold each head still and shelter the panicle with your body.
As the season progresses, you may also start to see the black midge parasitoid, Eupelmus spp. Whilst it can be present in reasonably large numbers, this parasitoid does not occur early enough to prevent midge from causing damage. It can also be confused with the midge, so be sure to look for the reddish abdomen of the midge, not just little black ‘flies’.
Managing midge in sorghum
Insecticides only kill the adult midge as they move about the crop and do not kill the eggs or hatched larvae that are already present inside the sorghum florets. While midge adults only live for one day, they do most of their egg laying (and subsequent damage to the crop) in the morning. Use the economic threshold calculator for sorghum midge to determine if treatment is warranted.
Synthetic pyrethroids and carbamates are the only effective products currently registered for sorghum midge control. Note that they are highly disruptive to beneficial insects that provide control of helicoverpa eggs and larvae, aphids and Rutherglen bug.
Rutherglen bug
Rutherglen bug (RGB) reduce grain yields by feeding on the developing seed. Crops are most susceptible to crop loss if RGB are present through early grain fill. Once grain reaches physiological maturity, RGB impact is negligible. Sorghum seed crops are particularly vulnerable to RGB damage as feeding not only reduces yield, but significantly reduces the viability (germination) of seed.
It is critical that RGB adult numbers are recorded if they are detected whilst sampling for midge and/or helicoverpa during flowering and grain fill. In some seasons, repeated immigration of RGB adults can make decisions about the need for, and timing of control, quite difficult.
Recent research on the efficacy of insecticides on RGB control has shown that synthetic pyrethroids have good knockdown control, but limited residual efficacy. Targeting RGB adults early, before they lay eggs may give a better control outcome than waiting until there are larger populations of adults and nymphs. RGB females start to lay eggs once the sorghum starts filling grain. RGB adults and nymphs will be incidentally controlled by applications of insecticide for sorghum midge.
RGB damage
RGB feeding directly on developing and filling grain reduces sorghum yield and quality by reducing grain weight and allowing entry of common fungi and bacteria that further deteriorates the grain. Infestations of RGB present from flowering can prevent grain from filling. This very early damage looks very much like midge damage. Infestations that occur once grain is filling (milky-hard) will result in grain that is heavily spotted with feeding injury to the seed coat and lighter in weight.
Heat can also impact on sorghum heads causing darkened and pinched grain. This type of damage may be confused with RGB feeding damage. The presence of feeding spots on the seed is characteristic of RGB-damaged grain.

Sorghum head that has not sustained RGB damage during early grain fill, but is now infested with adults; showing full grain and no spotting of seed

Developing sorghum head showing signs of RGB damage; undeveloped seed, and spotting on developing seed.
Sampling
RGB are typically clumped in their distribution. When beating individual heads in a bucket, you will find heads with no, or very few RGB and others with hundreds. RGB release a chemical that results in aggregations of individuals. When sampling it is useful to consider:
- The proportion and age/maturity of heads infested
- Whether nymphs are present
- Infestation distribution: are there earlier areas of the crop that are more heavily infested?
Infestations are typically initiated by movement of adults into the crop (often with winds associated with storms), so the youngest heads in the crop are at greatest risk of yield loss from prolonged RGB infestation.
Nymphs
RGB females lay eggs in the sorghum heads once they have fed on developing grain. RGB eggs and nymphs develop relatively slowly. Eggs take around 4-5 days to hatch and then the nymphs will take 2-3 weeks to develop from 1st instar to adult. This is why nymphs are generally only seen in maturing heads. Heavy infestations of nymphs will move up and down on the plant, feeding on leaves, stem, and seed heads. Very small nymphs cannot damage seed, but larger nymphs (3rd-5th instar) can cause damage. Nymphs do not appear to move from plant to plant, so uninfested heads near heavily infested ones will not automatically be infested.
Thresholds
If the crop has predominantly adults present, the economic thresholds are:
- Flowering to soft dough: 20-25 bugs per head
- Hard dough to harvest: no impact on yield.
Large populations of adults and nymphs are usually seen in heads that are starting to colour, through to harvest. Decisions about whether these later infestations warrant treatment should take into account the stage of grain maturity (physiologically mature (i.e. black layer) seed is not at risk).
For grain that is still soft dough:
- treat if adult numbers are over threshold
- if adult numbers are below threshold but nymph numbers above threshold, then treatment is warranted, but can be delayed if nymphs are small. This may allow time for the crop to reach physiological maturity.
Also consider the potential for large populations to cause harvest and delivery issues (clogging, excess moisture, delivery of live insects).
Control options
Reinfestation by adults is common in RGB. Checking treated fields at 2-3 days after spraying will help determine if the treatment was effective. Longer re-check intervals may be too long to distinguish between poor efficacy and reinfestation.
When RGB densities are extreme, it is challenging to get high levels of control. Keep in mind that 90% control of 400 RGB per head will still leave 40 RGB per head. Nymph populations can often be much higher than this.
The challenge of good control is exacerbated by large populations of nymphs that may be moving up and down the plant, and thus more difficult to contact directly with insecticide. For this reason, insecticides with some residual may be more effective against large, mobile populations of nymphs.