Presently there are few control options other than the use of chemical insecticides (or biopesticides), for above-threshold populations of larvae. Spraying should be carried out promptly once the threshold has been exceeded (see the Beatsheet’s threshold calculators for helicoverpa in sorghum, chickpeas and pulses).
Controlling helicoverpa effectively with insecticides depends on knowing which species are present in the crop. Helicoverpa punctigera is easily killed by all registered products, including products to which H. armigera is increasingly resistant.
On this page:
- Resistance management strategies
- Spray smart
- Monitoring
- Control without insecticides
- Natural enemies
- Managing overwintering pupae
- Whole-farm or regional approach
- More information
- See also:
Resistance management strategies
Industry dependence on insecticides for helicoverpa management has imposed severe selection pressure resulting in resistance to a range of products by H. armigera. With continued applications, the frequency of resistant individuals within a population increases, leading to field control failures.
H. armigera has a well-documented capacity to develop resistance to widely used insecticides. Insecticide resistance management strategies (IRMS) are available for both the grains and cotton industries:
- The grains IRMS is available from the IPM guidelines for grains website
- The cotton IRMS is available in the latest edition of the Cotton Pest Management Guide, on the CottonInfo website. The guide also contains a Resistance Management Plan for Bollgard 3.
Spray smart
Timing and coverage are both critical to achieving good control of helicoverpa larvae. Good coverage is essential for ingestion-active products because the larvae must actually feed on plant material covered with an adequate dose of the insecticide or biopesticide. Inappropriate timing risks crop loss and the costs of re-treating the field and increases the likelihood of insecticide resistance by exposing larvae to sub-lethal doses of insecticide.
Due to the resistance that helicoverpa has developed to major chemical groups, registered chemicals will not necessarily give adequate control in every situation. However, newer products for helicoverpa management often do not have an immediate ‘knockdown’ effect – larvae can take several days to die (but usually stop feeding soon after ingestion of the product). The presence of larvae still in the field post-spray is therefore not automatically an indicator of spray failure. Local knowledge of which chemicals are working in a particular area should be sought from consultants/agronomists.
Attract and kill products such as Magnet® consist of a liquid moth lure based on floral volatiles mixed with an insecticide. Only a relatively small area needs to be treated (less than 2% of the total crop), minimising impact on natural enemies. Reducing the pest moth population decreases the number of eggs laid into a crop, which can lower subsequent pest pressure and delay the need for foliar insecticides.
Monitoring
Regular crop scouting enables assessment of both the number and relative ages of helicoverpa larvae in the crop .
- Early detection is critical to ensure effective timing of sprays.
- Timing sprays for when larvae are feeding or moving in the open will make them more easily contacted by spray droplets than when they have moved into protected feeding locations (e.g. flowers, cobs, pods or bolls).
- Ensure larvae are at an appropriate size to control effectively with the intended product. Very small and small (up to 7 mm) larvae are the most susceptible stages, however they grow rapidly. Reassess if a spray application is delayed more than 2 days.
- Assess the economic damage (i.e. only spray if the value of the crop saved is more than the cost of spraying). Vegetative feeding generally does not equate to significant yield loss.
Control without insecticides
Other management tools include:
- Insect-resistant genetically modified (GM) cotton has been developed to produce a highly specific toxin from a naturally occurring bacterium called Bacillus thuringiensis (or Bt) that kills helicoverpa larvae when ingested. GM cotton has significantly reduced insecticide use, however use of the technology involves license fees and compliance costs.
- Natural enemies. Be aware of the presence of beneficial arthropods and pathogens in the crop, and factor their likely impact into management decisions.
- Pupae busting can reduce both the size of overwintering H. armigera populations and the carryover of insecticide-resistant individuals from season to season. Pupae busting can also have an impact on weed populations.
- Removing alternate hosts in and around crops can prevent the build-up of Helicoverpa and other insect pests.
- Spring trap crops have been successfully used as an area-wide management tool for reducing the size of the overall helicoverpa population as it emerges from diapause in spring.
Natural enemies
Predators
Many predators are opportunity feeders that also feed on prey other than helicoverpa, and some may have preferences for a particular helicoverpa life stage (e.g. larvae or eggs). The most common predators of helicoverpa in field crops are:
- predatory bugs (e.g. spined predatory shield bug, assassin bug, and damsel bug)
- predatory beetles (e.g. ladybirds, red and blue beetle, carab beetle, and soldier beetle)
- spiders
- lacewings (green and brown)
- ants.
Parasitoids
Some wasps and flies attack helicoverpa eggs, larvae or pupae. Parasitoids that attack helicoverpa larvae do not kill their hosts immediately, but stop or slow down caterpillar feeding, reducing the impact of the pest on the crop. Groups most active in field crops include:
- small wasp species such as Microplitis, Trichogramma and Telenomus
- relatively large parasitoid wasps (Netelia, Heteropelma, Ichneumon)
- flies (Carcelia and Chaetopthalmus).
Pathogens
Many naturally occurring diseases infect and kill helicoverpa larvae, including nucleopolyhedrovirus (NPV) and fungal pathogens (Metarhizium, Nomuraea and Beauveria). Another disease – ascovirus – stunts larval development, and is spread by wasp parasitoids.
Two pathogens that affect helicovrpa are available commercially as biopesticides:
- Helicoverpa NPV is a highly selective product that is harmless to humans, wildlife and beneficial insects.
- Bt is a bacterial toxin from Bacillus thuringiensis available as a selective spray that only kills moth larvae. Genes from the Bt organism have also been used to genetically modify cotton plants so that the toxin is expressed in the plant’s tissues. When young helicoverpa larvae feed on a Bt cotton plant, the toxin kills susceptible individuals.
Conserving natural enemies
Natural enemies will rarely eradicate all eggs or larvae, but may reduce infestations to below economic threshold if predators and parasitoids are not disrupted by broad-spectrum insecticides. The amount of disruption that insecticides cause to natural enemy activity varies depending on which chemicals are used and which natural enemies are active.
Managing overwintering pupae
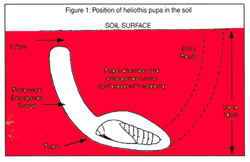
Diagram of helicoverpa pupal chamber
Overwintering describes the winter dormant stage of the helicoverpa pupae (also referred to as diapause). Overwintering populations are almost entirely Helicoverpa armigera, and are the source of resistance between seasons.
Shorter day lengths (<12 hours) and declining temperatures (<12°C) induce larvae to enter diapause. Prior to pupating, larvae form a pupal chamber as well as an emergence tunnel for moths to exit.
In southern Queensland and northern New South Wales, helicoverpa start to enter diapause from early March onwards. Earlier pupations emerge as moths in autumn or winter. In southern regions these moths rarely survive or breed successfully. However, in Central and North Queensland, a significant proportion of moths can successfully breed throughout winter due to higher temperatures and suitable crop or weed hosts.
Moth emergence commences in spring in response to increasing temperatures. In northern regions this can start in late August while in cooler southern regions emergence may not start until October. Moths generally emerge over a six week period, but a small proportion continues to emerge through to December and occasionally January.
Structural integrity of the emergence tunnel is vital for successful moth emergence. Cultivation up to a depth of 10 cm (‘pupae busting’) before the end of August dramatically reduces pupal survival by either killing them directly, exposing them to predators, or disrupting the emergence tunnel (thus trapping them in the chamber). Pupae busting is most effective when there has been high numbers of large larvae in a late season crop.
Whole-farm or regional approach
There is no simple solution to helicoverpa control in a farming system that provides a wide range of food sources throughout the year. Area-wide management strategies are designed to manage helicoverpa at a regional level rather than each farmer making helicoverpa control actions in isolation. It requires high levels of communication and cooperation between farmers, consultants, and research/extension personnel.
More information
