The surveillance program and its benefits
Each year the NSW Department of Primary Industries conducts a helicoverpa insecticide resistance surveillance program in the major summer cropping regions of NSW and Queensland, focused on detecting helicoverpa resistance to key helicoverpa-selective insecticides.
The program provides growers and advisors with an early warning system for potential resistance outbreaks in the northern region, helps maximise the effectiveness of spray applications, and is essential for informing ongoing review and improvement of industry-endorsed resistance management strategies.
The program utilises F2 screening for detecting resistance to indoxacarb, emamectin benzoate and chlorantraniliprole which now have broad registration in pulses and are also registered for use of fall armyworm. This type of screening is highly sensitive for all types of known and novel resistance even when resistance genes are recessive.
This predictive capability means industries can implement management tactics for reducing economic losses well before spray failures occur, as well as minimising further spread of resistance genes throughout the wider H. armigera population.
F2 screening involves testing the grandchildren of moths from field populations to generate F2 progeny though a step-wise process shown in Figure 1.
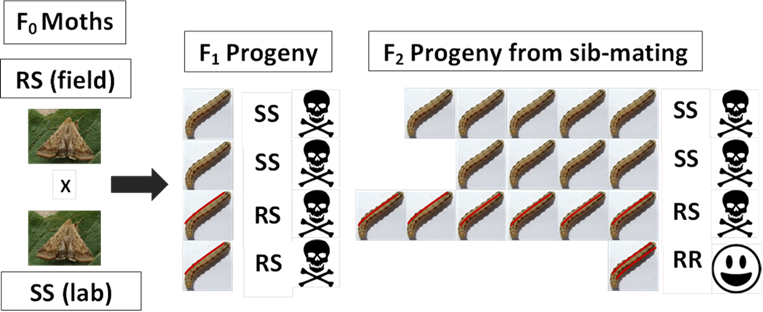
Figure 1. F2 screen for detection of resistance alleles. Moths are collected from pheromone traps and, in this example, one parent has one copy of the resistance gene (RS). Their F1 progeny are sib-mated to produce the F2 generation. If resistance is completely recessive then only 1 in 16 of the F2 progeny will be homozygous (RR) for the resistance gene and will survive a diagnostic dose of insecticide. The remaining susceptible (SS) and heterozygous (RS) progeny will be killed.
Summary of results from 2023-24 and comparison to long term trends
Emamectin benzoate
No insects tested positive for resistance to emamectin benzoate in 2023-24. This is consistent with the historical trend of very low resistance to this insecticide.
Chlorantraniliprole
No insects tested positive for resistance to chlorantraniliprole in 2023-24. However, in past seasons resistance to this insecticide was detected at levels higher than the industry average in the Darling Downs and Dawson Callide regions (Figure 2).
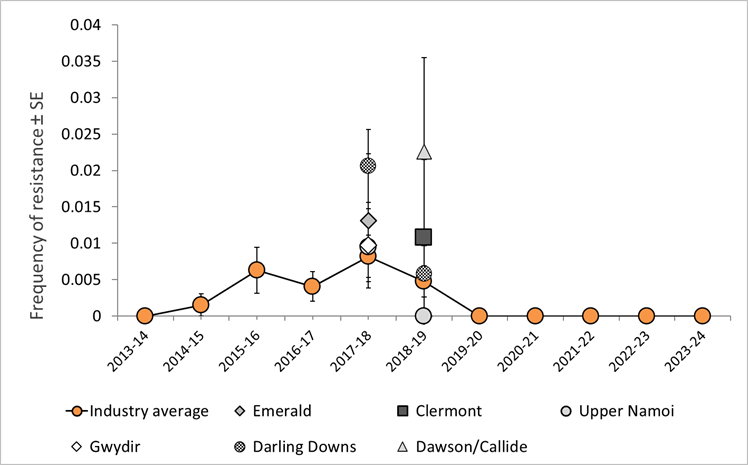
Figure 2. Annual frequency of H. armigera chlorantraniliprole resistance in all regions where resistance was detected compared with the industry average ± binomial standard error (SE).
Indoxacarb
Resistance to indoxacarb can vary significantly between regions and between seasons (see Table 1 and Figure 3).
- CENTRAL AND NORTHERN QLD: Resistance was higher than the industry average in all years up to and including 2018-19 where it peaked at 14% and was 2.4-fold above that in the southern regions. It then declined and has stablilised over the past three seasons with approximately 6% of the H. armigera population carrying genes for resistance in 2022-23. In 2023-24 resistance has increased again to 9% on average, ranging from 7% in the Emerald Irrigation Area to 16% in the Dawson/Callide region.
- SOUTHERN QLD AND BORDER RIVERS: The Darling Downs recorded a significant increase in indoxacarb resistance from approximately 6% in 2020-21 to 15% in 2021-22. In the 2022-23 season resistance remained higher than the industry average in the Darling Downs at 10%, with similar levels recorded in 2023-24 (11%).
Resistance in the St George and Mungindi regions was also higher than the industry average in 2022-23 at 12%. Resistance declined slightly this season to approx. 8% in the Border Rivers region. - NORTHERN NSW: Resistance in the Namoi and Gwydir valleys increased from 4.3% in 2021-22 to 7.3% in 2022-23. In 2023-24 resistance has increased again to 9%.
- CENTRAL NSW: In 2020-21 a significant increase in indoxacarb resistance was recorded in the Macquarie region when resistance peaked at 17% compared to 2017 when resistance levels were between 2-4%. In the following two seasons resistance declined from these historical levels to 7% and 4% in 2021-22 and 2022-23, respectively. In 2023-24 no indoxacarb resistance was detected in the Macquarie region.
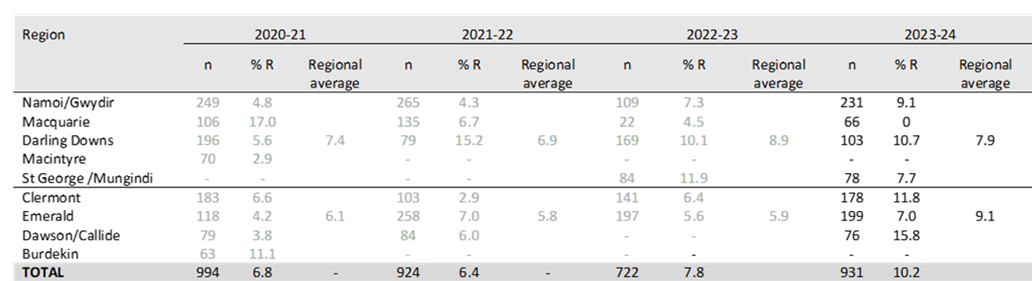
Table 1. Regional indoxacarb resistance in the past four seasons (n = number of tests).
Overall, in 2023-24 there has been a further incremental increase in industry-wide average resistance to this insecticide driven by higher frequencies in central Qld. This follows a continuing trend of resistance seen in eastern Australia prior to the drought of 2017-19 (Figure 3).
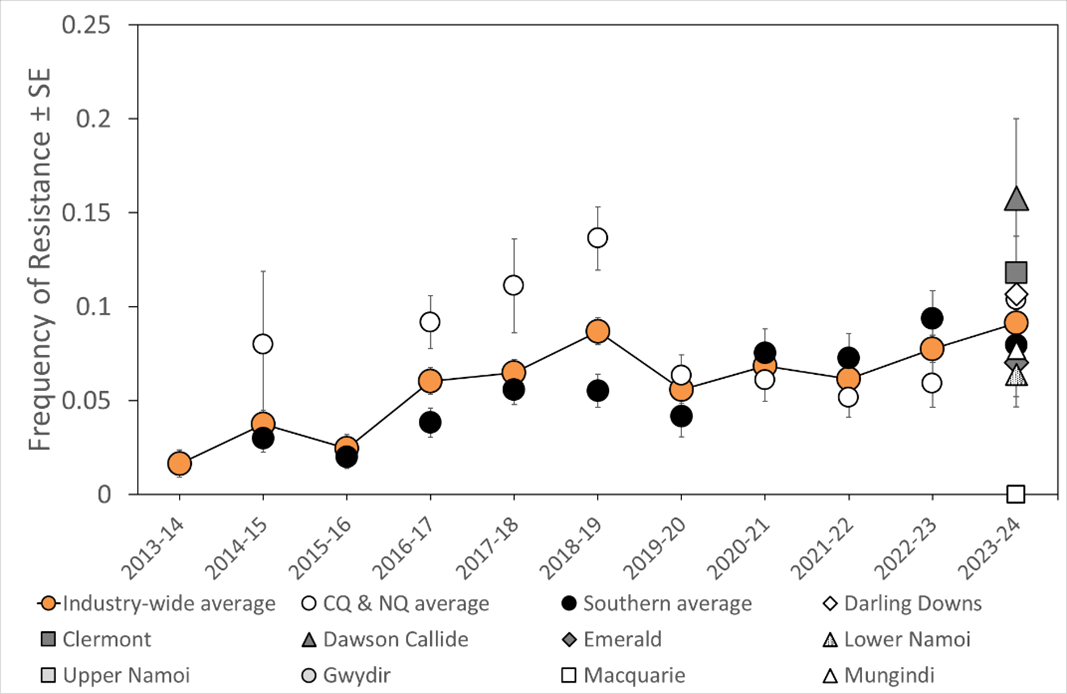
Figure 3. Annual frequency of H. armigera indoxacarb resistance in central Qld (CQ) and north Qld (NQ) compared with the industry and southern (southern Qld and NSW) averages ± binomial standard error (SE).
Management implications
The continued pattern of very low resistance to emamectin benzoate and chlorantraniliprole is a positive sign that these insecticides will continue to provide effective control of helicoverpa. Stabilisation of indoxacarb resistance in southern Queensland is also encouraging. However, there are signs of selection for resistance to this insecticide re-emerging in central Queensland.
Patterns of variability in different seasons and in different regions are likely to reflect localised insecticide usage patterns. Increased spraying for fall armyworm may have also increased selection pressure in maize, sweetcorn and sorghum where H. armigera is also a pest.
Therefore, it is important to adopt industry best practices for resistance management, particularly during the peak spray periods for helicoverpa in winter and summer pulses to minimise the risk of resistance building to dangerous levels that could result in spray failures.
To reduce the risk of lost productivity due to resistance, growers and agronomists are urged to consult the Resistance Management Strategy (RMS) in grains for key recommendations. The strategy is based on best practice product use windows and restrictions on the number of sprays to minimise selection pressure from the same chemical group across consecutive generations of H. armigera. The use of a broad range of IPM options will reduce over-reliance on any one chemical group. Following the strategy’s recommendations and complying with label instructions will minimise the risk of spray failures and support sustainable management of H. armigera.
General principles to minimise resistance development:
- Comply with all directions on product labels – DO NOT cut rates or exceed the recommended applications per crop per season.
- Avoid repeated use of insecticides from the same chemical group; if a spray fails due to resistance or unknown cause, do not re-spray using that group in the same season.
- Monitor regularly, using appropriate sampling techniques.
- Correctly identify the pest to ensure the most effective insecticide and rate is used.
- Monitor beneficial populations to determine if chemical control of helicoverpa is warranted.
- If available, use economic thresholds when making spray decisions.
- Avoid prophylactic sprays
- Where possible, use target-specific ‘soft’ chemicals rather than broad-spectrum pesticides.
- Consider the impact on all species present when applying insecticide sprays; be aware of potential implications for helicoverpa resistance when managing for fall armyworm.
- Ensure spray rigs are calibrated properly and sprays achieve effective coverage.
- Monitor post-treatment for evidence of loss of field efficacy and report field failures.
For more information:
Read the GRDC’s resistance management strategy for Helicoverpa armigera and check relevant spray windows. The science behind the Helicoverpa armigera RMS is available at IPM Guidelines for Grains.
Related Beatsheet articles:
- Is that larva dying, or still causing damage? Recognising the symptoms of newer insecticides on Helicoverpa larvae, March 2018.
- New strategy released to manage Helicoverpa resistance, July 2018.
- Unnecessary spraying not good for your crop, your industry, or your bank balance, Oct 2019.
This surveillance is supported by GRDC and CRDC through projects DPI2307-002RTX and DAN2601.
Page last updated May 2024.
