Helicoverpa outbreaks in wheat are relatively rare events. The last big outbreak, in 2016, was the first significant event for several decades, so treating helicoverpa in wheat is not something that many agronomists are familiar with. However, 2020 looks to be a year when helicoverpa infestations in some crops may warrant control. Wet winters are well correlated with outbreaks of H. armigera. The warm autumn also meant that a smaller proportion of H. armigera went into winter diapause, meaning higher levels of activity earlier in spring.
When the 2016 outbreak occurred, there were no thresholds to guide decisions about control, simply because these events had been so rare. In 2016, DAF Field Crop Entomology took the opportunity to do some basic trial work to try and determine an appropriate threshold, and associated guidance on sampling and control. Over the past few years, we have done some more detailed work to better understand the impacts.
Most importantly, the dominant species in wheat is Helicoverpa armigera which has developed resistance to the older insecticide groups that are registered for helicoverpa control in winter cereals. Consequently, targeting small larvae and expecting a lower level of control are considerations for maximising efficacy of spraying.
Early instar larvae feed on leaf material in the canopy, and medium-large larvae move up onto the heads and feed on grain that is milky-soft. Larvae tend to only graze on more mature grain.
Larval sampling
Whilst the beat sheet is an efficient way of sampling larvae in the crop, the graph below illustrates how deficient this method is for dislodging small larvae, compared with medium-large larvae. Only 10% of the small and very small (VS-S) larvae present in the crop were dislodged on to the beat sheet. In comparison, 80% of medium-large larvae were dislodged. This result has major implications for the timely application of insecticides targeting smaller larvae.
Whilst not widely used in the northern region, the sweep net is another useful and rapid sampling method, particularly for shorter crops. We compared the relative efficacy of the beat sheet and sweep net, and found that the sweep net was an even poorer method of estimating VS-S larvae (only detecting 40% of what the beat sheet detected), but the sweep net was a better method for detecting medium-large larvae. The sweep net potentially catches a greater proportion of the larvae it dislodges, whilst larvae can be ‘lost’ (i.e. flung off plants across rows) when sampling with the beat sheet.
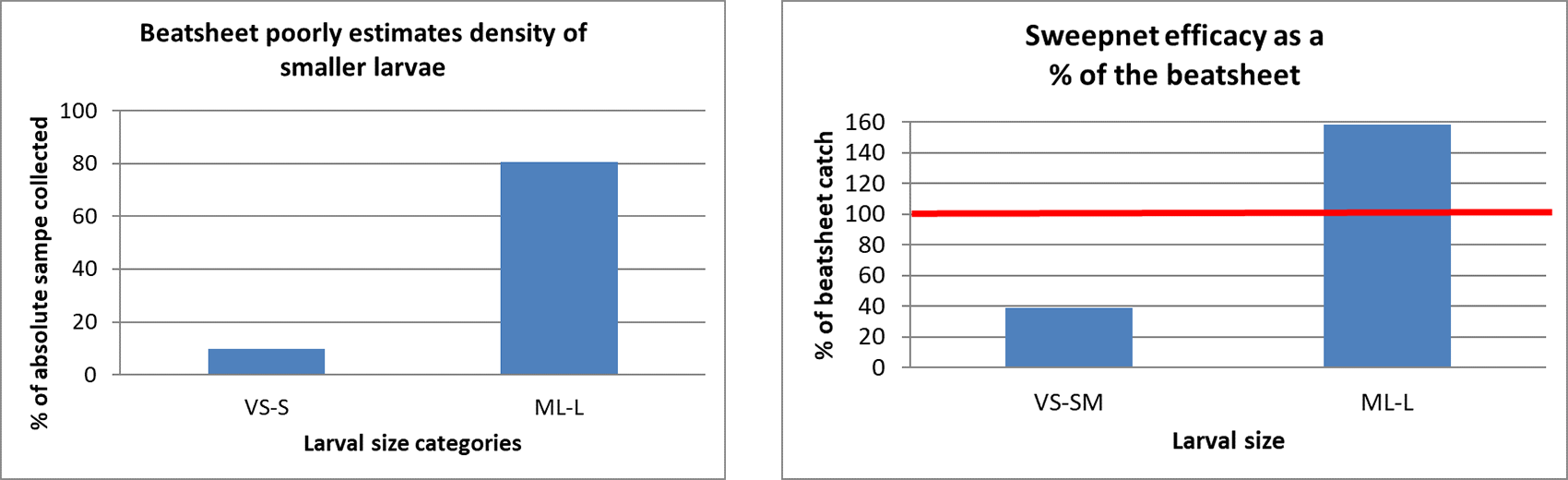
Figure 1. Sampling efficiency of beat sheet versus sweep net of two sizes of larvae. The number of larvae ‘caught’ with the beatsheet was compared with an absolute sample of the crop where metres of row were cut and searched for all larvae.
Estimating yield loss
Only one field trial has been undertaken to assess the impact of helicoverpa on wheat, so the recommendations are preliminary.
The yield loss is estimated to be 42 kg/ha for every larva per sqm (based on an absolute larval count).
This yield loss estimate is considerably higher than the grain loss estimates for helicoverpa feeding directly on grain in other crops (e.g. chickpeas or sorghum = 24-25 kg/ha), and it is not entirely clear why this is so.
What the field trial, and subsequent controlled trials has shown is that helicoverpa partially consume a high proportion of grain, resulting in more losses at harvest and a high defective grain risk. For these reasons, a conservative approach to helicoverpa infestations in winter cereals is suggested.
Whilst direct feeding on grain has been the focus of trial work to date, it is worth considering the potential impact of defoliation on yield. In 2020, CSIRO used the wheat model in APSIM to model the potential impact of defoliation (notionally by armyworm) in wheat. The following is an extract from the report of the investigation.
The general plasticity of wheat plants allows them to tolerate significant levels of defoliation across most growth stages. However, defoliation between floral initiation and flowering can significantly reduce yield. This is a well-recognised susceptible period in a crop’s development (Dreccer et al., 2018; Kirkegaard et al., 2018; Lake et al., 2016) and the whole concept of identifying critical periods for flowering (Flohr et al., 2017; Lilley et al., 2019) is based on the reduction of stress during this time. The sensitivity of this stage is evident when relatively small defoliation events cause a reduction in yield while large events are catastrophic. Generally, vegetative wheat plants can tolerate defoliation even benefitting from it when the defoliation spares resources for later in the season. This is a common outcome when using crops for a dual grain and graze purpose (Bell et al., 2015; Sprague et al., 2014; Tian et al., 2012). However, once the critical period is achieved, significant yield losses will occur.
Is there a point at which helicoverpa larvae are no longer able to damage grain?
Examination of helicoverpa capacity to damage maturing grain shows that medium larvae (and therefore most likely large as well) will feed on grain even when it is mature and drying.
The following graph illustrates the damage potential of 4th instar larvae fed grain at different stages of maturity. There is a decline in the amount of grain consumed only at the latest stage of maturity offered (Z92), but even at this maturity stage, some feeding damage occurs.
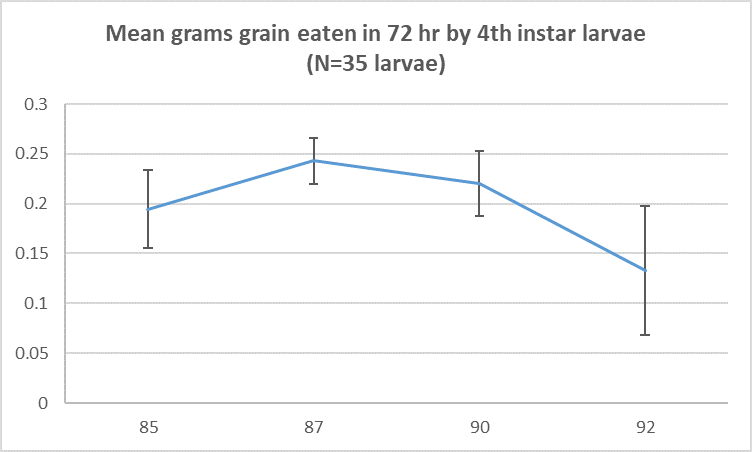
Figure 2. Grams of grain eaten by a 4th instar larva at four developments stages Z85, Z87, Z90 and Z92. Visual representation of the grain stages presented below.

(pictured left to right: grain at Z85, Z87, Z90 and Z92.)
Speed of larval development
Modelling can be used to estimate how quickly the larvae will develop in your area at a particular time of year. Below is output from the DARABUG2 model, using Dalby as an example. A 4th instar helicoverpa found on 26 October (highlighted), resulted from an egg laid in early October and will pupate in early November. The risk of grain loss, caused by helicoverpa feeding, will be caused by 4th-6th instar larvae.
| stage | Stage_duration | Time_start | Time_end |
|---|---|---|---|
| egg | 7.166667 | 6/10/2019 | 13/10/2019 |
| L1 | 4.708333 | 13/10/2019 | 18/10/2019 |
| L2 | 5.083333 | 18/10/2019 | 23/10/2019 |
| L3 | 3.458333 | 23/10/2019 | 26/10/2019 |
| L4 | 1.458333 | 26/10/2019 | 28/10/2019 |
| L5 | 2.458333 | 28/10/2019 | 30/10/2019 |
| L6 | 4.583333 | 30/10/2019 | 4/11/2019 |
| pupa | 18.29167 | 4/11/2019 | 22/11/2019 |
| adult | 1.541667 | 22/11/2019 | 24/11/2019 |
The DARABUG model is available on the Beatsheet. Model outputs can be downloaded as a csv file to provide output like that presented above.
Armyworms in winter cereals
Relatively low densities of native armyworm species are being found in winter cereals, and there have been no reports of head lopping. Armyworm are most easily distinguished from helicoverpa by the 3 white stripes on the collar (band just behind the head capsule – see Figure 3), which may/may not continue longitudinally down the body.

Figure 3. Armyworm larvae in a sweep net. The white circles show where the location of the white strips on the collar, just behind the head – visible on 3 of the larvae. Uncurling larvae over a finger will better expose the collar for confident identification.
Armyworms are defoliators, interested only in leaf material, but will graze on awns and glumes as leaf quality declines. As long as there is green leaf available in the crop, armyworms will feed on it, and high densities can be tolerated in the crop. Head lopping occurs typically when the foliage dries off, and only the nodes on the stems remain green. Medium – large armyworm larvae will feed on this last bit of green material, resulting in the severing of the stem and loss of the head.
Death of armyworm larvae as a result of parasitism is common. Cotesia sp (a wasp parasitoid) is one of the most common and recognisable natural enemies attacking armyworm. Other common natural enemies are predatory bugs and parasitic flies.

Figure 4. Armyworm larva (centre) killed by the parasitoid Cotesia sp. On the right are the remnants of the Cotesia larval cocoons, formed by the parasitoid larvae as they emerged from the armyworm. On the left are the Cotesia wasps which emerged from the cocoons (reared though in the lab).
Risk of fall armyworm in cereals
Whilst it is possible that FAW could lay eggs in winter cereals, and larvae develop, it is unlikely that in reproductive crops there will be sufficient time for the larvae to cause significant crop loss. Again, FAW is principally a defoliating species and literature suggests that it is less damaging to maturing grain than helicoverpa would be.

Thanks for spotting that Jeff. Photo is fixed.
I think the photo of wheat with Zadok stages should be rotated.