Helicoverpa armigera active now.
This spring a number of agronomists have just started monitoring pheromone traps across the northern region, and the results from the past two weeks illustrate how useful pheromone traps can be.
Over the past 1-2 weeks, the traps are catching relatively high numbers of Helicoverpa armigera, and low catches of H. punctigera. In September it is generally assumed that any helicoverpa larvae in chickpea crops are H. punctigera because H. armigera do not start emerging from diapause until late September at the earliest.
The presence of H. armigera in cropping areas at this time of year sounds a warning for those considering using a synthetic pyrethroid to control helicoverpa larvae in their winter pulses. At this point we only have information for a small number of traps, and only for the past 1-2 weeks. We cannot discount the possibility that there were earlier flights of H. punctigera, and that the larvae in the crop are not H. armigera. However, Peter Gregg has been running traps in Western Qld through the winter and was catching reasonable numbers of H. punctigera until mid August when the trap catches dropped off.
Although we can’t be definitive about the species mix of larvae in chickpea crops now, the likelihood is that a proportion of larvae will be H. armigera. Consequently, the potential for control failure if using an SP is higher than in seasons when H. armigera are not present. Even if only small numbers of H. armigera are present, the use of SPs will promote resistance in these populations.
Using the helicoverpa development model, it is possible to estimate when the eggs were laid, which have generated the larvae in crops now. I have run the model for Dalby and St George, and the predictions are shown in the table below.
Results of ‘backcasting’ larval development to determine when moths may have been laying in chickpeas.
Why is H. armigera active so early?
The Helicoverpa emergence model is predicting H. armigera will start to emerge from diapause in late September through to early Novermber (depending on where you are). It is likely that the H. armigera moths being seen are emerging locally, and are doing so now because they were not in diapause. In autumn, helicoverpa pupae go into diapause if the temperatures are sufficiently low to trigger diapause. Through March-May, an increasing proportion of the pupae go into diapause as temperatures decline. If temperatures are warmer, fewer pupae will diapause.
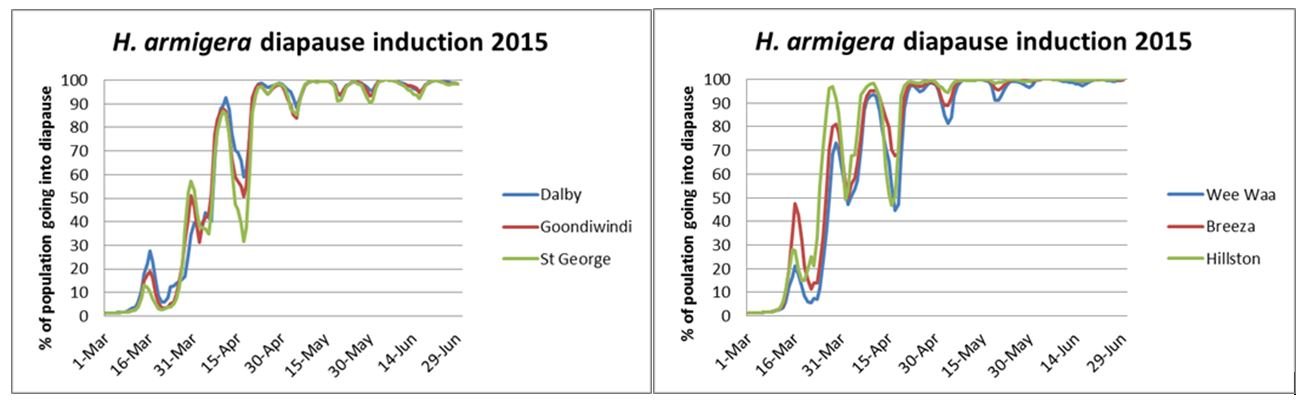
The graphs below illustrate how the proportion of the population going into diapause is influenced by fluctuations in temperature. The cooler the temperatures are in the week leading up to pupation, the more likely the pupa is to go into diapause. On any one day, a proportion of the population goes into diapause, and the rest do not. As temperatures become more consistently cool, the larger the proportion of larvae going into diapause.
In the autumn of 2015, in the Qld regions, no more than 50% of the pupae forming before the end of March were going into diapause. In NSW regions, there was a high proportion of pupae predicted to go into diapause in late March. In both regions there were short periods of warmer conditions in April where the percentage of the pupating helicoverpa going into diapause dropped.
Other insect pest activity to watch for:
Aphids in winter cereals
There has been lot of discussion around whether to control aphids in winter cereals through August and into September. As the season progresses, the need to control generally decreases as the aphid populations start to decline. With warming temperatures, the natural enemy activity increases, particularly the parasitoid wasps which can have a big impact on the populations. Once you start to see parasitised aphids (brown mummies) it is likely that the aphid population will slow down in terms of size.
Armyworm in winter cereals.
There have been a few reports from northern NSW and the western Downs of armyworm activity in crops. Use a beatsheet to check for larvae in the crop, and be sure to check the ground along the rows for larger larvae that are sheltering during the day. These are the ones that are going to do damage, so you want to know how many you have got. Armyworm don’t always lop heads, but the risk is highest when there are large larvae in crops that are drying down.
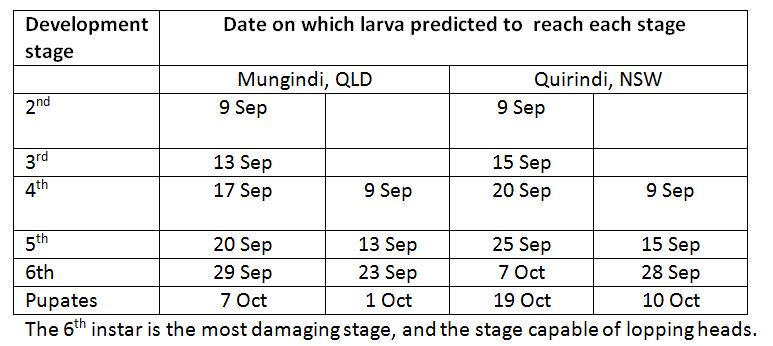
Garry McDonald (cesar, Melbourne Uni) has resurrected the program that allows us to predict armyworm development. Running the model allows us to look forward and see what stage the larvae will be when the crop is most susceptible to head lopping (when drying down).
I have run the model for Mungindi and Quirindi, forecasting likely rate of development for two stages of larvae seen in the field on 9 September. Keep in mind that these predictions are based on long term average temperatures, and if the actual conditions are significantly cooler, the rate of development will slow, and warmer conditions will speed it up.
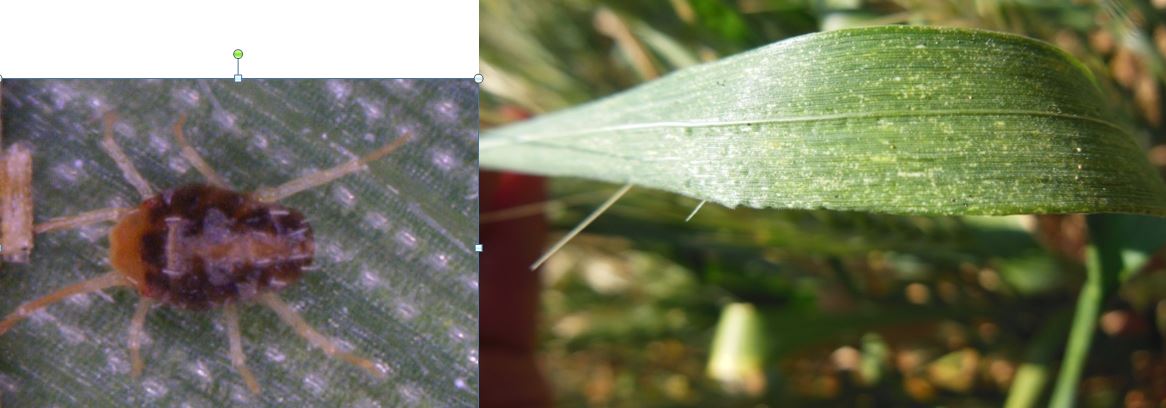
Brown wheat mite may occur in western areas where conditions are warm and dry.
The brown wheat mite is tiny, and it’s presence is typically detected because of plant symptoms. Infested crop looks generally unthrifty, and may start to brown on the leaf margins. Close inspection of plants will show mottling of the leaves where the mites have fed, and tiny mites running about. The impact of the mites will be evident in the response of the crop. Treatment may be warranted if there is significant damage to the green leaf area of the upper leaves. Organophosphates are registered for brown wheat mite control.